Hypertropin by NeoGenica
PRODUCT NAME
Generic name: Recombinant Human Growth Hormone for Injection
Trade name:
Chinese Phoneticize: Zhusheyong Chongzu Ren Shengzhangjisu
Composition in effect: Recombinant Human Growth Hormone
BRIEF INTRODUCTION
Ansomone is a kind of sterile, lyophilized formulation of Recombinant Human Growth Hormone (HGH) with 191 amino acids, derived from engineering E.coli, and it is identical to the natural growth hormone in amino acid sequence and three-dimension structure. Ansomone is indicated for growth failure due to endogenous growth hormone deficiency (GHD) and Turner disease or Kidney failure. can be used to heal up the surgery wound or burned wound and it has good effect to preservation for human aging. Our manufacturing plants of are certified for GMP Standard by SFDA.
PHARMACOLOGY AND TOXICOLOGY
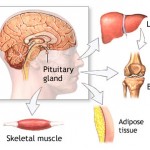
Ansomone® exerts the same actions of endogenous human growth hormone. It can stimulate proliferation and differentiation of epiphysis chondrocyte, stimulate growth of cartilage matrix cells, stimulate proliferation and differentiation of osteoblast; thus accelerate the liner growth rate and improve epiphysis width. Ansomone® can promote protein synthesis in whole body; reverse the negative nitrogen equilibrium caused by wound and surgery; correct the hypoproteinemia due to severe infection or hepatocirrhosis; stimulate synthesis of immune globin and proliferation of lymphadenoid, macrophage and lymphocyte, thus enhance the ability of infection resistance; stimulate proliferation of collagenocyte, fibroblast and macrophage in sites of burn and surgery, thus accelerate wound healing; promote synthesis of cardiocytes, thus improve cardiac contractility and reduce cardiac oxygen consumption; regulate lipometabolism, thus depress serum cholesterol and low density lipoprotein’s level; complement insufficiency or deficiency of growth hormone, regulate adult’s lipometabolism, osteometabolism, heart and kidney function.
PHARMACOKINETICS
It is reported that the equal pharmacological effect could be achieved via subcutaneous (sc) or intramuscular (IM) administration. Even though sc may lead a higher concentration of GH in plasma, IM could also yield the same IGF-I level. The absorption of GH is the relatively slow, Cmax often occurs at 3-5 hours after injection. Clearance of GH is via liver and kidney, the half-life of clearance is about 2-3hours. Uncatabolized GH excreted in urea is almost immeasurable. All of the GH in circulation system exists as a complex form with GH binding proteins that make the half-life of GH prolonged.
INDICATIONS
Ansomone® is indicated for the growth failure of children due to endogenous growth hormone deficiency (GHD), chronic renal insufficiency (CRI) and Turner’s syndrome. Ansomone® is indicated for severe burn.
More information
USAGE
When reconstitution, 1ml water for injection should be injected along the bottle wall, then swirl the vial with a gentle rotary motion until the contents are completely dissolved, do not shake violently.
The recommended dosage of Ansomone® for the treatment of children growth failure is 0.1~0.15IU/kg daily subcutaneous administration for 3 months to 3 years. Therapy regimen could be modified according to experienced doctor’s suggestion. The recommended dosage of Ansomone® for the treatment of severe burn is 0.2~0.4IU/kg daily subcutaneous administration for 2 weeks.
ADVERSE REACTIONS
Growth hormone may cause transient hyperglycemia; it can be recovered as the administration proceeded or terminated.
Adverse reactions occurred in about 1% short stature children in clinical trial. Common adverse reactions include slight pain, tingle, turgescence around the injection site and peripheral edema, arthralgias. All of those adverse reactions often occurred at the beginning of treatment, and were temporal and tolerable. Long-term and high dosage administration of hGH may develop the antibodies in a few patients. However, the antibodies concentration could be rarely up to as high as 2mg/L that might affect the therapeutic efficiency.
CONTRAINDICATIONS
1. Ansomone® should not be used in children whose epiphysis had been closed.
2. Ansomone® should not be used in cancer patients.
3. Ansomone® should not be used in patients in acute shock stage with severe infection.