Serono’s Saizen (Serostim and Zorbtive)
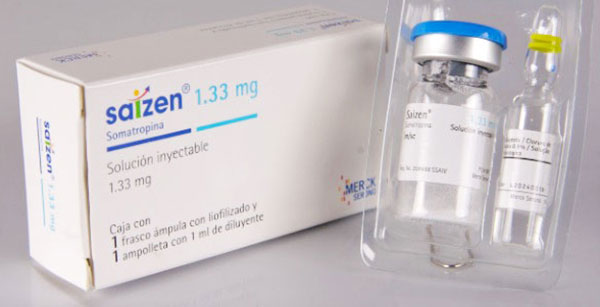
Saizen was developed by Switzerland’s Serono. It is available for sale in both the U.S. and Europe. Saizen is manufactured by Mouse-cell technology. In the U.S., the Food and Drug Administration (FDA — an agency that regulates the medicine market) has approved Saizen for treatment of pediatric and adult GHD, growth failure in females with Turner’s syndrome, and growth failure caused by chronic renal disease. Saizen’s cool.click™ is a needle-free device that delivers the medication through the skin.
The also produce Serostim:
Serostim was also developed by Serono. It is available for sale in the U.S. Serostim is manufactured by Mouse-cell technology. In the U.S., the FDA has approved Serostim to treat muscle wasting in AIDS and cachexia patients. Serostim is available in lyophilized powder and is delivered by use of syringe.
Also mage by Serono is Zorbtive:
Zorbtive was developed by Serono. It is available for sale in the U.S. Zorbtive is manufactured by Mouse-cell technology. In the U.S., the FDA has approved Zorbtive to treat growth retardation due to short bowel syndrome. Zorbtive is available in lyophilized powder and is delivered by use of syringe.